Drug metabolism is the entire process of how drugs are transformed and converted from one chemical form to another. That process begins the moment a substance enters our bodies and does not end until it is excreted.
➤ Get your medical card now with The Cannigma Medical
Sometimes people talk about this metabolism process as the body “breaking down” chemicals, but in most cases metabolism actually does the opposite — adding in more molecules to the chemical structure. As it turns out, often the larger the chemical becomes, the easier it is to eliminate it from your system.
In general, drug metabolism occurs in the liver, due to the presence of large amounts of enzymes. These enzymes serve as a major filtration system for the body, protecting us from toxins in the environment. But metabolism doesn’t take place exclusively in the liver, and drug metabolism can take place in every biological tissue in the human body. Still, while these other sites are involved to a more limited extent, the liver is the primary organ responsible for metabolism — especially for things that we eat or take by mouth.1
The drug metabolism itself takes place in two different phases:
In phase 1, enzymes initiate a chemical reaction that typically oxidize the drug, increasing its water solubility. While this phase might produce compounds that are easy to eliminate from the body, many chemicals aren’t ready for elimination yet.
In phase 2, these modified chemical compounds undergo another round of chemical reactions, where the drug is usually linked to a larger, more excretable molecule. Catalyzed by another class of enzymes, called transferase enzymes or UTG’s, this transformation causes the chemicals to become water soluble — and thus able to be eliminated from the body.
Activating cannabis in the body
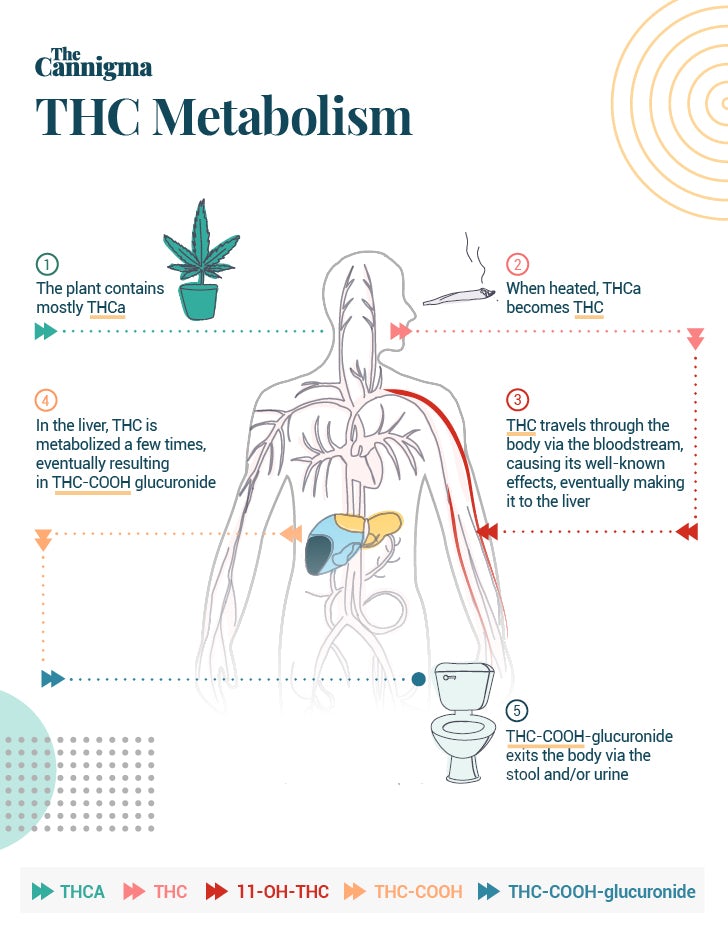
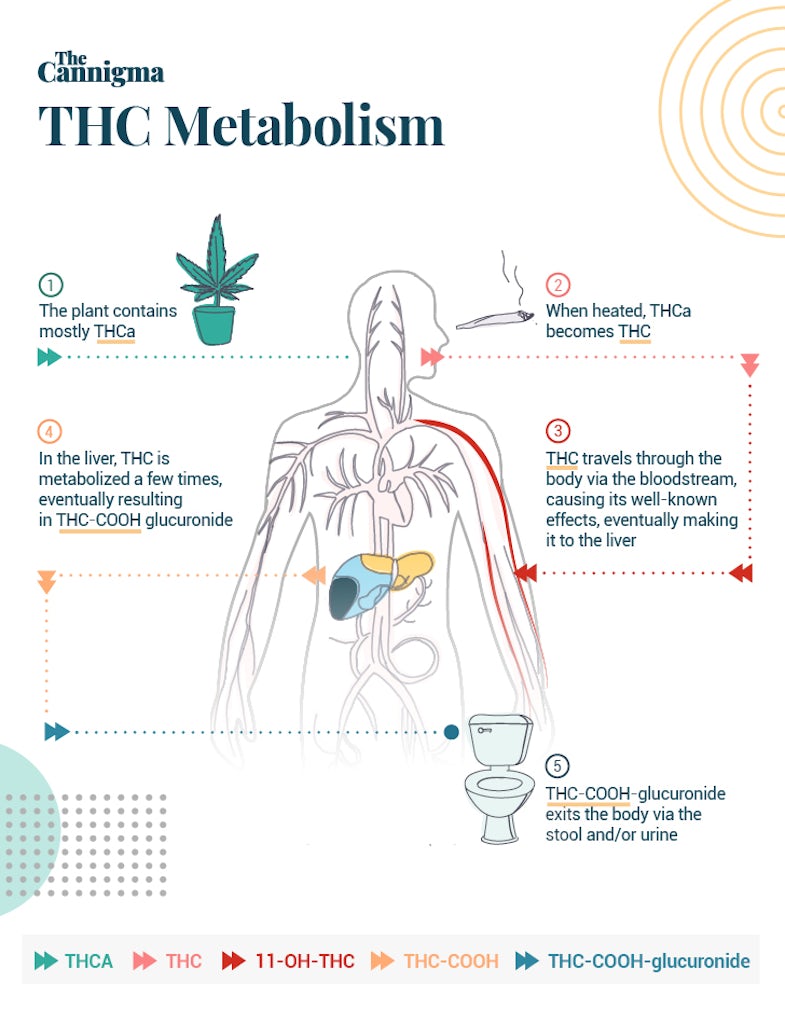
All cannabinoids actually begin life in an acid form. In raw cannabis flowers, like those used for smoking, vaping or making edibles, there isn’t much THC or CBD. Instead we find THCA and CBDA — their acidic precursors.2
While these cannabinoid acids sometimes have active medicinal effects, they tend to be markedly different from their well-known neutral forms. THCA, for example, shares THC’s anti inflammatory effects but produces no psychoactive high.3
THC becomes active when THCA is transformed through a process called decarboxylation. Decarboxylation usually occurs with heat, so it happens naturally when you burn or heat cannabis to smoke or vaporize it. The same is true for CBDA and the other minor cannabinoids. Decarboxylation is also an important step in making cannabis edibles at home, and is often referred to as “decarbing” or “activating” cannabis.
How does THC metabolism begin?
Once cannabinoids like THC have been consumed, absorbed into the bloodstream, and distributed to tissues throughout the body, they can begin to cause their desired effects.
The path THC takes through your body is actually dictated by how you consume it. If inhaled, the cannabinoids and terpenes travel rapidly from the lungs to the blood and brain. But if you ingest THC, the path to the blood and brain is not so simple. Cannabis taken orally must first be absorbed from the intestines and sent through the liver. Any cannabinoids sent to the liver, will be metabolized by your liver cells — a process known as first-pass metabolism.4
While the liver is the primary location that metabolic transformations take place (due to the high level of enzymes), cannabinoid metabolism can also take place in other tissues. For example, it’s been shown experimentally that THC can be metabolized in the brain.5
While there are many different ways cannabinoid metabolism can happen, much like other drugs, it commonly takes place in the following two phases:
THC Metabolism Phase 1: The creation of 11-OH-THC
Phase 1 metabolization is all about hydroxylation and oxidation. In this phase, particular enzymes hydroxylate part of the cannabinoid, adding an oxygen and hydrogen molecule to its structure.6
With THC this leads to the creation of 11-OH-THC, a very pharmacologically active metabolite, known for its sedative and psychoactive effects.
Interestingly, we see lower levels of 11-OH-THC in blood plasma when someone has smoked cannabis than we do when cannabis is eaten. This is primarily because THC first passes through the digestive system when eaten, which means it first passes through the liver. Therefore with edible cannabis, more of the THC is processed by the liver and converted to 11-OH-THC before making its way into the bloodstream.7

When 11-OH-THC is oxidized by the same family of enzymes, it produces THC-COOH, an inactive metabolite which is one of the main end products of cannabis use. Usually by 30-45 minutes after smoking, THC-COOH concentrations have gradually increased and are found in higher levels than THC concentrations.
While 11-OH-THC is a highly active metabolite in the body, THC-COOH is considered an inactive metabolite, meaning it is no longer able to bind to cannabinoid receptors and is thought to be without medicinal effects. THC-COOH is what drug tests look for to detect the presence of cannabis in urine, and this inactive metabolite can be stored for over 30 days.8
Other cannabinoids such as CBD go through similar processes, undergoing metabolization into a variety of metabolites. Just like THC, the process for metabolism of CBD can vary depending on the consumption method. Although notably, CBD is not well absorbed and some of the drug passes through the system unchanged and is excreted in feces.
Interestingly, the enzymes responsible for phase 1 metabolization can vary from person to person due to genetic factors. So some people metabolize THC and CBD differently from others. Some experience the psychotropic effects longer than others, or are more likely to fail a drug test due to a higher accumulation of cannabinoids in their system.9
THC Metabolism Phase 2: Cannabis exits the body
In phase 2, the process is all about preparing the drug to exit the body. In this step UGT enzymes are able to connect a glucuronide molecule to THC-COOH. This turns the chemical into a THC-COOH-glucuronide molecule, which is easy to excrete from the body in substances like urine and fecal matter. Once transformed to this metabolite, the drug is ready to be eliminated from the body.10
In the last stage of this process, the metabolites of THC are eliminated from the body when the person urinates or has a bowel movement.
Within an average of five days, 80-90% of THC and (mostly) its metabolites will have been excreted — more than 65% via feces and 20% via the urine. But this isn’t the end of the story. Some of the THC metabolites will remain bound up in tissue, and will be released more slowly. This is why THC’s metabolites can be detected weeks after use for some cannabis users, and typically longer for those who have excess body fat (THC-COOH storage space).11
As we can see, the active components in cannabis take a long journey from absorption to elimination — transforming from one chemical to another as they go. What starts out as THCA before we smoke it, is THC when we first absorb it, and will likely end up as a totally different chemical by the time it exits your body.
Even more amazing is that this process is very different depending on route of administration, and so are the effects. Still, this incredible process enables cannabis to enter our system, provide its impressive array of effects, and then exit our system without building up — making medical cannabis use possible.
Sources
- Iyanagi T. (2007). Molecular mechanism of phase I and phase II drug-metabolizing enzymes: implications for detoxification. International review of cytology, 260, 35–112. https://doi.org/10.1016/S0074-7696(06)60002-8
- Arno Hazekamp, Justin T. Fischedick, Mónica Llano Díez, Andrea Lubbe, Renee L. Ruhaak, 3.24 – Chemistry of Cannabis, Editor(s): Hung-Wen (Ben) Liu, Lew Mander,
Comprehensive Natural Products II, Elsevier, 2010, Pages 1033-1084, ISBN 9780080453828, https://doi.org/10.1016/B978-008045382-8.00091-5 - Nallathambi, R., Mazuz, M., Ion, A., Selvaraj, G., Weininger, S., Fridlender, M., Nasser, A., Sagee, O., Kumari, P., Nemichenizer, D., Mendelovitz, M., Firstein, N., Hanin, O., Konikoff, F., Kapulnik, Y., Naftali, T., & Koltai, H. (2017). Anti-Inflammatory Activity in Colon Models Is Derived from Δ9-Tetrahydrocannabinolic Acid That Interacts with Additional Compounds in Cannabis Extracts. Cannabis and cannabinoid research, 2(1), 167–182. https://doi.org/10.1089/can.2017.0027
- Huestis M. A. (2007). Human cannabinoid pharmacokinetics. Chemistry & biodiversity, 4(8), 1770–1804. https://doi.org/10.1002/cbdv.200790152
- Harvey, D. J., & Martin, B. R. (1979). Marijuana: Biological Effects. Nahas, GG.; Paton, WDM., editors.
- Sharma, P., Murthy, P., & Bharath, M. M. (2012). Chemistry, metabolism, and toxicology of cannabis: clinical implications. Iranian journal of psychiatry, 7(4), 149–156.
- Schwilke, E. W., Schwope, D. M., Karschner, E. L., Lowe, R. H., Darwin, W. D., Kelly, D. L., Goodwin, R. S., Gorelick, D. A., & Huestis, M. A. (2009). Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clinical chemistry, 55(12), 2180–2189. https://doi.org/10.1373/clinchem.2008.122119
- Huestis M. A. (2007). Human cannabinoid pharmacokinetics. Chemistry & biodiversity, 4(8), 1770–1804. https://doi.org/10.1002/cbdv.200790152
- Sachse-Seeboth, C., Pfeil, J., Sehrt, D., Meineke, I., Tzvetkov, M., Bruns, E., Poser, W., Vormfelde, S. V., & Brockmöller, J. (2009). Interindividual variation in the pharmacokinetics of Delta9-tetrahydrocannabinol as related to genetic polymorphisms in CYP2C9. Clinical pharmacology and therapeutics, 85(3), 273–276. https://doi.org/10.1038/clpt.2008.213
- Huestis M. A. (2007). Human cannabinoid pharmacokinetics. Chemistry & biodiversity, 4(8), 1770–1804. https://doi.org/10.1002/cbdv.200790152
- Huestis M. A. (2007). Human cannabinoid pharmacokinetics. Chemistry & biodiversity, 4(8), 1770–1804. https://doi.org/10.1002/cbdv.200790152
Sign up for bi-weekly updates, packed full of cannabis education, recipes, and tips. Your inbox will love it.

 Shop
Shop Support
Support